Welcome to edspace.american.edu. This is your first post. Edit or delete it, then start blogging!
Autopilot
DECEMBER 12, 2019
Jonny Saunders from Michael Wehr’s lab at the University of Oregon recently posted a preprint documenting their project Autopilot, which is a python framework for running behavioral experiments:
Autopilot is a python framework for behavioral experiments through utilizing Raspberry Pi microcontrollers. Autopilot incorporates all aspects of an experiment, including the hardware, stimuli, behavioral task paradigm, data management, data visualization, and a user interface. The authors propose that Autopilot is the fastest, least expensive, most flexibile behavioral system that is currently available.
The benefit of using Autopilot is that it allows more experimental flexibility, which lets researchers to optimize it for their specific experimental needs. Additionally, this project exemplifies how useful a raspberry pi can be for performing experiments and recording data. The preprint discusses many benefits of raspberry pis, including their speed, precision and proper data logging, and they only cost $35 (!!). Ultimately, the authors developed Autopilot in an effort to encourage users to write reusable, portable experiments that is put into a public central library to push replication and reproducibility.
For more information, check out their presentation or the Autopilot website here.
Additionally documentation is here, along with a github repo, and a link to their preprint is here.
Oat: online animal tracker
December 5, 2019
Jonathan Newman of the Wilson Lab at Massachusetts Institute of Technology has developed and shared a set of programs for processing video.
The only thing you’ll enjoy more than an oat milk latte from your favorite coffeeshop is the Oat collection of video processing components designed for use with Linux! Developed by Jonathan Newman of Open Ephys, this set of programs is useful for processing video, extracting object position information, and streaming data. While it was designed for use with real-time animal position tracking, it can be used in multiple applications that require real-time object tracking. The individual Oat components each have a standard interface that can be chained together to create complex dataflow networks for capturing processing, and recording video streams.
Read more about Oat on the Open Ephys website, or check out more on Github!
Touchscreen Cognition and MouseBytes
NOVEMBER 21, 2019
Tim Bussey and Lisa Saksida from Western University and the BrainsCAN group developed touchscreen device chambers that can be used to measure rodent behavior. While the touchscreens themselves are not an open-source device, we appreciate the open-science push for creating a user community, performing workshops and tutorials, and data sharing. Most notably, their sister project, MouseBytes, is an open-access database for all cognitive data collected from the touchscreen-related tasks:
Touchscreen History:
In efforts to develop a cognitive testing method for rodents that would optimally reflect a touchscreen testing method in humans, Bussey et al., (1994, 1997a,b) developed a touchscreen apparatus for rats, which was subsequently adapted for mice as well. In short, the touchscreens allow for computer-aided graphics to be presented to a rodent and the rodent can make choices in a task based on which stimuli appear. The group published a “tutorial” paper detailing the behavior and proper training methods to get rats to perform optimally using these devices (Bussey et al., 2008). Additionally, in 2013, three separate Nature Protocols articles were published by this group, with details on how to use the touchscreens in tasks assessing executive function, learning and memory, and working memory and pattern separation in rodents (Horner et al., 2013; Mar et al., 2013; Oomen et al., 2013).
Most recently, the group has developed https://touchscreencognition.org/ which is a place for user forums, discussion, training information, etc. The group is actively doing live training sessions as well for anyone interested in using touchscreens in their tasks. Their twitter account, @TouchScreenCog, highlights recent trainings as well. Through developing automated tests for specific behaviors, this data can be extrapolated across labs and tasks.
Additionally, MouseBytes is an open-access database where scientists can upload their data to, or can analyze other data already collected from another group. Not only does this reduce redundancy of experiments, but also allows for transparency and reproducibility for the community. The site also performs data comparison and interactive data visualization for any data uploaded onto the site. There are also guidelines and video tutorials on the site as well.
Nature Protocols Tutorials:
Original Touchscreen Articles:
Bussey, T. J., Muir, J. L., & Robbins, T. W. (1994). A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neuroscience Research Communications, 15(2), 103-110.
You can buy the touchscreens here.
Editor’s Note: We understand that Nature Protocols is not an open-access journal and that the touchscreens must be purchased from a commercial company and are not technically open-source. However, we appreciate the group’s ongoing effort to streamline data across labs, to put on training workshops, and to provide an open-access data repository for this type of data.
The OpenMV project: Machine Vision with Python
November 14, 2019
OpenMV – Better, Stronger, Faster and only $65 USD
Recent updates to the firmware for the OpenMV H7 Camera have brought some new functionality to this device, which is popular for open source neuroscience projects (e.g. Rodent Arena Tracker, or RAT: https://hackaday.io/project/162481-rodent-arena-tracker-rat). The new firmware allows for use of the popular TensorFlow library for machine learning on this MicroPython-based device. It’s small (1.5 by 1.75 inches), consumes only a max of 140 mA when processing data, has 1 MB of RAM and 2 MB of flash, and runs 64-bits computations at 4800 MHz (3.84 GB/s bandwidth). OpenMV is capable of frame differencing, color tracking, marker tracking, face detection, eye tracking, person detection (with TensorFlow Lite), and more. The project supports a very easy to use GUI, the OpenMV IDE. It’s intuitive to use, and offers a number of ready to go applications. Arduino users will feel right at home, despite the code being Python based.

Check out the project here: https://openmv.io/.
An automated behavioral box to assess forelimb function in rats
November 7, 2019
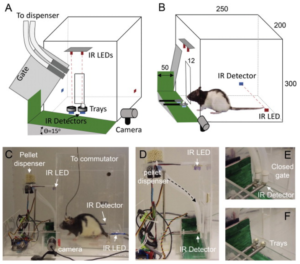
Chelsea C. Wong and colleagues at the University of California – San Francisco have developed and shared a design for an open-source behavioral chamber for the measurement of forelimb function in rats.
Forelimb function (reaching, grasping, retrieving, etc) is a common readout of behaviors for studying neural correlates of motor learning, neural plasticity and recovery from injury. One version of the task used commonly to study these behaviors, the Whishaw single-pellet reach-to-grasp task, traditionally requires an experimenter to manually present each pellet and to shape the behavior rats by placing a subsequent pellet only when the rat has relocated to the other end of the cage over multiple trials. Wong et al. developed an open source, low-cost, automated high-throughput version of this task. The behavioral apparatus, constructed out of commercially available acrylic sheets, features a custom built pellet dispenser, cameras and IR detectors for measuring position of a rat and position of a pellet, and an Arduino board to integrate information about the animal with dispensing of the pellet. Code for automation of the task was built in MATLAB and includes a GUI for altering experiment parameters. Data collected can be analyzed using MATLAB, excel, or most other statistical programming languages. The authors provide example data from the device to highlight its potential use for combining this reaching task with chronic electrophysiological recording techniques. The full design is available in their publication in Journal of Neuroscience Methods.
 Check out the full publication here!
Check out the full publication here!
Automated Home-Cage Rodent Two-bottle Choice Test: open-source success story
October 31, 2019
Elizabeth Godynyuk and colleagues from the Creed Lab at Washington University, St. Louis recently published their design for a two-bottle choice homecage apparatus in eNeuro. It incorporates the original design (published on Hackaday.io in May 2018), modifications from Jude Frie and Jibran Khokar (Frie & Khokhar, 2019), and additional improvements over the course of use. This project is a great example of collaborative open-source tool development.
Studies of liquid ingestive behaviors are used in neuroscience to investigate reward-related behavior, metabolism, and circadian biology. Accurate measurement of these behaviors are needed when studying drug administration, preference between two substances, and measuring caloric intake. To measure consummatory behavior in mice between two liquids, members of the Creed lab designed a low-cost and arduino-based device to automatically measure consumption in a homecage two-bottle choice test. Posted to Hackaday in May 2018, the initial version of the device used photointerrupters to measure time at the sipper, 15 mL conical tubes for volumetric measurements of fluid, and a 3D printed holder for the apparatus. Data from the photobeams are recorded to an SD card using a standard Arduino. In August 2018, the project was updated to Version 2, to make it battery powered and include a screen to display data. They made the editable TinkerCAD design available on hackaday.io.
In October 2018, Dr. Jibran Khokhar and colleagues at the University of Guelph posted a project log highlighting the modifications making the device larger and suitable for studying liquid intake in rats. This updated design was published in April 2019 in HardwareX. This device gives the advantage of being able to analyze the drinking microstructure by recording licking behavior and volume consumed in real time. Modifications include larger liquid reservoirs and adding a hydrostatic depth sensor, allowing each bout of drinking to correspond to a specific change in volume.
In current day, Elizabeth Godynyuk and colleagues from the Creed lab have shared their own updated version of the device in eNeuro. It remains low-cost and open-course and results validating the device with preference testing are shared. Furthermore, the authors show that the two-bottle choice test apparatus can be integrated with a fiber photometry system. In the eNeuro article, Godynuyuk et al. cite Frie and Khokhar’s modifications to highlight how the design can be easily adjusted to fit investigator needs.
These two projects show how open source projects can be modified and how different groups can collaborate to improve upon designs. This shows how open source projects allow research groups can modify designs to best address their research questions instead of forming their research questions based on the commercial tools available.
Creed Lab Version 1: https://hackaday.io/project/158279-automated-mouse-homecage-two-bottle-choice-test
Creed Lab Version 2: https://hackaday.io/project/160388-automated-mouse-homecage-two-bottle-choice-test-v2
Frie and Khokar 2019 (HardwareX): https://www.sciencedirect.com/science/article/pii/S2468067219300045#b0005
Godynyuk et al 2019 (eNeuro): https://www.eneuro.org/content/6/5/ENEURO.0292-19.2019.long
SfN 2019 Open-Source Itinerary
October 18, 2019
It’s that time of year again: SfN season! The OpenBehavior Team has put together a curated itinerary of talks and posters featuring open-source tools. The list contains over 200 projects related to open-source in general, with 90 or so projects that are specifically relevant to behavioral neuroscience (denoted in bold)! You can find it at this link: https://docs.google.com/document/d/1s09KorOuBcga2TygATB6lXcjpNYlMxCEZ1yXzxWsnYE/edit?usp=sharing.
Thanks to everyone who reached out to add their projects to the itinerary! If you want to add your poster or talk to the list, it’s not too late! Send us a DM on twitter or a quick email to openbehavior@gmail.com.
See you in Chicago!
Updates on LocoWhisk and ART
OCTOBER 3, 2019
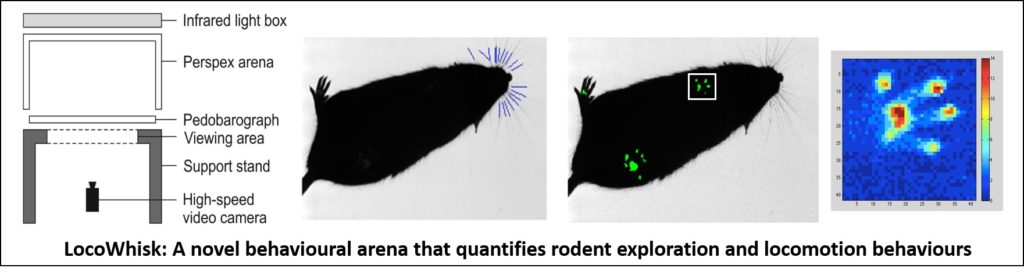
Dr Robyn Grant from Manchester Metropolitan University in Manchester, UK has shared her group’s most recent project called LocoWhisk, which is a hardware and software solution for measuring rodent exploratory, sensory and motor behaviours:
In describing the project, Dr Grant writes, “Previous studies from our lab have shown that that analysing whisker movements and locomotion allows us to quantify the behavioural consequences of sensory, motor and cognitive deficits in rodents. Independent whisker and feet trackers existed but there was no fully-automated, open-source software and hardware solution, that could measure both whisker movements and gait.
We developed the LocoWhisk arena and new accompanying software, that allows the automatic detection and measurement of both whisker and gait information from high-speed video footage. The arena can easily be made from low-cost materials; it is portable and incorporates both gait analysis (using a pedobarograph) and whisker movements (using high-speed video camera and infrared light source).
The software, ARTv2 is freely available and open source. ARTv2 is also fully-automated and has been developed from our previous ART software (Automated Rodent Tracker).
ARTv2 contains new whisker and foot detector algorithms. On high-speed video footage of freely moving small mammals (including rat, mouse and opossum), we have found that ARTv2 is comparable in accuracy, and in some cases significantly better, than readily available software and manual trackers.
The LocoWhisk system enables the collection of quantitative data from whisker movements and locomotion in freely behaving rodents. The software automatically records both whisker and gait information and provides added statistical tools to analyse the data. We hope the LocoWhisk system and software will serve as a solid foundation from which to support future research in whisker and gait analysis.”
For more details on the ARTv2 software, check out the github page here.
Check out the paper that describes LocoWhisk and ARTv2, which has recently been published in the Journal of Neuroscience Methods.
LocoWhisk was initially shared and developed through the NC3Rs CRACK IT website here.
3D Printed Headcap and Microdrive
SEPTEMBER 26, 2019
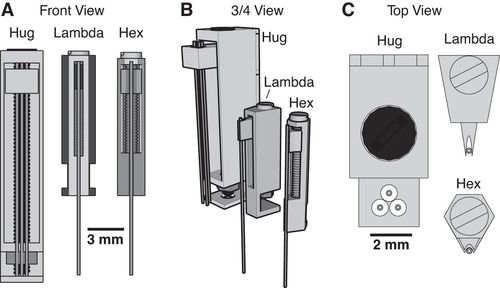
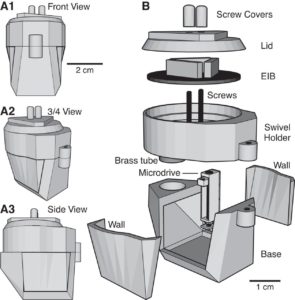
In their 2015 Journal of Neurophysiology article, the Paré Lab at the Center for Molecular and Behavioral Neuroscience at Rutgers University describe their novel head-cap and microdrive design for chronic multi-electrode recordings in rats through the use of 3D printing technology and highlight the impact of 3D printing technology on neurophysiology:
There is a need for microdrives and head-caps that can accommodate different recording configurations. Many investigators implant multiple individual drives aiming to record from numerous areas. However, this extends surgery time, impairs animal recovery, and complicates experiments. Other strategies rely on more expensive custom-machined drive assemblies that are specifically built for a particular set of regions, limiting their adaptability. Some proposed designs allow targeting of multiple regions, but recording sites must be within a few millimeters so are only suitable for mice and not for accessing areas of larger brains (like in rats, for example).
Utilizing 3D printing technology to create a novel design concept of microdrives and head-caps, this group’s design allows for recording of multiple brain regions in different configurations. In their article, the lab reviews the basic principles of 3D design and printing and introduce their approach to multisite recording, explaining how to construct the individual required components. The 3D printed head cap and electrode microdrive enables investigators to perform chronic multi-site recordings in rats. The head cap is composed of five components and there are three types of microdrives that can be used in different combinations or positions to study different targets. The different microdrive designs have different functionality including for extended driving depths, targeting of thin layers, and allowing many microdrives to be placed in a small area.
To show the viability of their new designs, the lab presents LFP recordings obtained throughout the cortico-hippocampal loop using 3D printed components. The lab suggests investigators modify their designs to best suit their research needs and give changeable versions of the three parts most important in modification. The investigators also provide a detailed explanation of the printing, assembly, and implantation of the head caps and microdrives. Finally, they indicate the ways 3D printing advancements can change how chronic implants are designed and used, notably 3D scanning and new material development.
For more information on the microdrive and headcap, see their paper’s Appendix, which has full instructions and advice on building these devices.
Headley, D. B., DeLucca, M. V., Haufler, D., & Paré, D. (2015). Incorporating 3D-printing technology in the design of head-caps and electrode drives for recording neurons in multiple brain regions. Journal of Neurophysiology, 113(7), 2721–2732. https://doi.org/10.1152/jn.00955.2014