SEPTEMBER 19, 2019
Richard Warren, a graduate student in the Sawtell lab at Columbia University, recently shared his new open-source project called SignalBuddy:
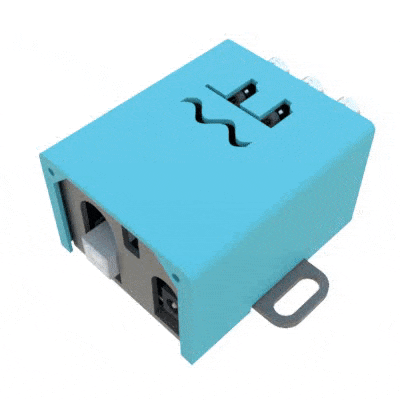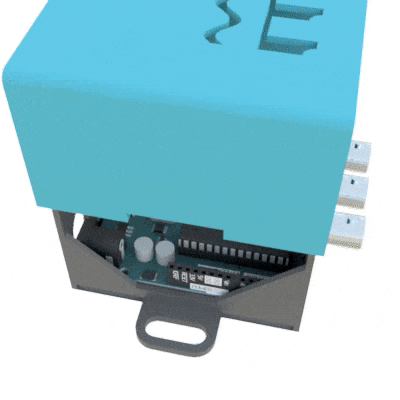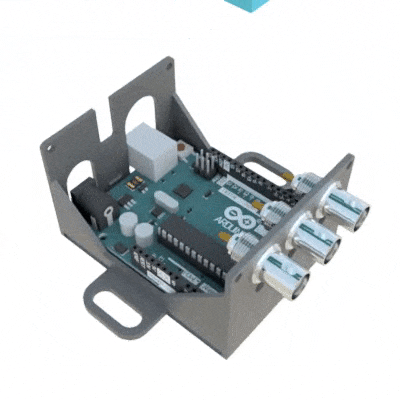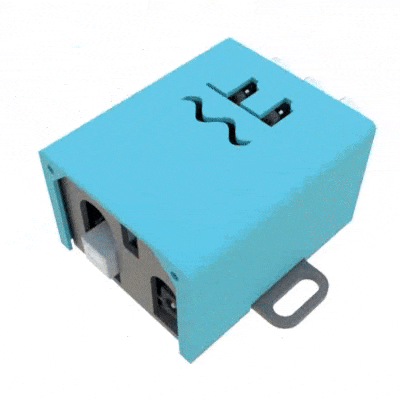
SignalBuddy is an easy-to-make, easy-to-use signal generator for scientific applications. Making friends is hard, but making SignalBuddy is easy. All you need is an Arduino Uno! SignalBuddy replaces more complicated and (much) more expensive signal generators in laboratory settings where one millisecond resolution is sufficient. SignalBuddy generates digital or true analog signals (sine waves, step functions, and pulse trains), can be controlled with an intuitive serial monitor interface, and looks fabulous in an optional 3D printed enclosure.
To get SignalBuddy working, all you need to do is install the SignalBuddy.ino Arduino code provided on their github, and follow the step-by-step instructions on github to get the Arduino programmed up for your specific experimental needs. SignalBuddy can be used for numerous lab purposes, including creating pulse trains for optogenetic light stimulation, microstimulation, electrophysiology, or for programming up stimuli for behavioral paradigms.
Additionally, their hackaday site provides the instructions for 3D printing an enclosure to house the Arduino inside using just two .stl files.
For more information, check out the SignalBuddy github repository here.
You can also get further details on the SignalBuddy Hackaday.io page here.
Fun Fact: This group also developed KineMouse Wheel, a project previously posted on OpenBehavior and is now being used in numerous labs! Cheers to another great open-source project from Richard Warren and the Sawtell lab!