SEPTEMBER 26, 2019
In their 2015 Journal of Neurophysiology article, the Paré Lab at the Center for Molecular and Behavioral Neuroscience at Rutgers University describe their novel head-cap and microdrive design for chronic multi-electrode recordings in rats through the use of 3D printing technology and highlight the impact of 3D printing technology on neurophysiology:
There is a need for microdrives and head-caps that can accommodate different recording configurations. Many investigators implant multiple individual drives aiming to record from numerous areas. However, this extends surgery time, impairs animal recovery, and complicates experiments. Other strategies rely on more expensive custom-machined drive assemblies that are specifically built for a particular set of regions, limiting their adaptability. Some proposed designs allow targeting of multiple regions, but recording sites must be within a few millimeters so are only suitable for mice and not for accessing areas of larger brains (like in rats, for example).
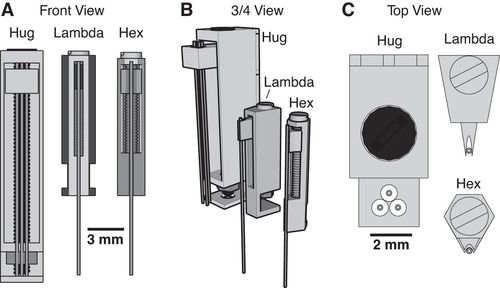
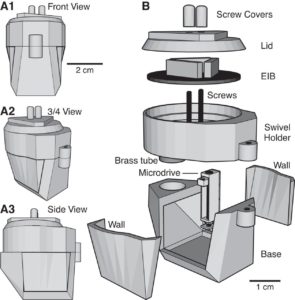
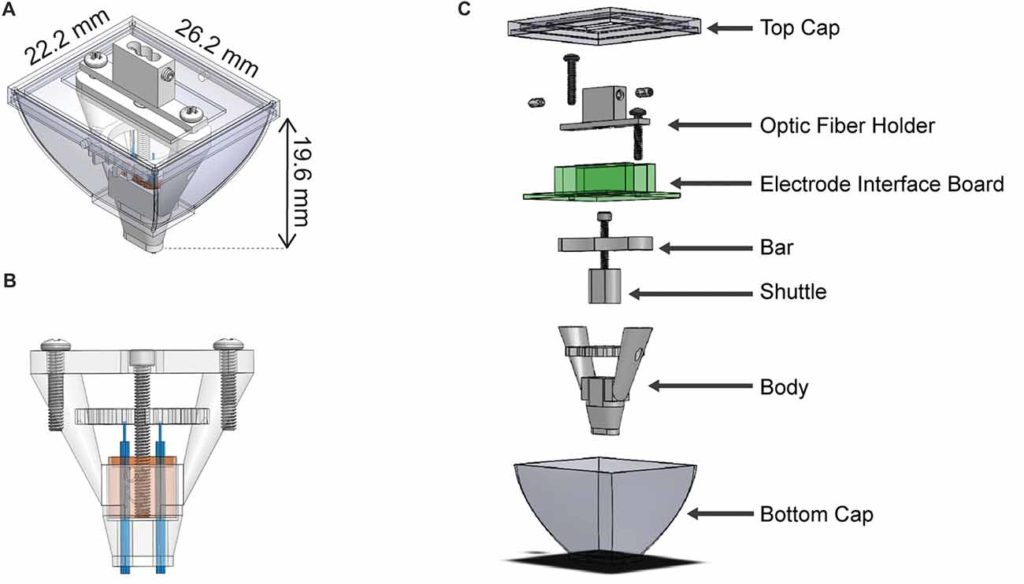
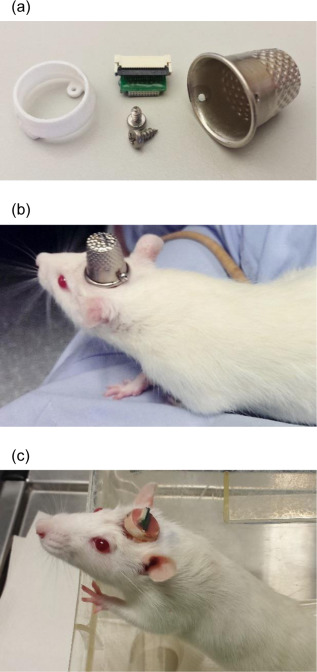
Utilizing 3D printing technology to create a novel design concept of microdrives and head-caps, this group’s design allows for recording of multiple brain regions in different configurations. In their article, the lab reviews the basic principles of 3D design and printing and introduce their approach to multisite recording, explaining how to construct the individual required components. The 3D printed head cap and electrode microdrive enables investigators to perform chronic multi-site recordings in rats. The head cap is composed of five components and there are three types of microdrives that can be used in different combinations or positions to study different targets. The different microdrive designs have different functionality including for extended driving depths, targeting of thin layers, and allowing many microdrives to be placed in a small area.
To show the viability of their new designs, the lab presents LFP recordings obtained throughout the cortico-hippocampal loop using 3D printed components. The lab suggests investigators modify their designs to best suit their research needs and give changeable versions of the three parts most important in modification. The investigators also provide a detailed explanation of the printing, assembly, and implantation of the head caps and microdrives. Finally, they indicate the ways 3D printing advancements can change how chronic implants are designed and used, notably 3D scanning and new material development.
For more information on the microdrive and headcap, see their paper’s Appendix, which has full instructions and advice on building these devices.
Headley, D. B., DeLucca, M. V., Haufler, D., & Paré, D. (2015). Incorporating 3D-printing technology in the design of head-caps and electrode drives for recording neurons in multiple brain regions. Journal of Neurophysiology, 113(7), 2721–2732. https://doi.org/10.1152/jn.00955.2014